Corona Virus FAQs
With the first case of corona virus being reported in NYC on 3/1/2020, here are some quick FAQs for all New Yorkers. Be sure to check out www.cdc.gov for latest updates.
WHAT IS THE CORONA VIRUS?
A novel virus, known technically as SARS-CoV-2, is responsible for causing a flu-like disease called COVID-19.
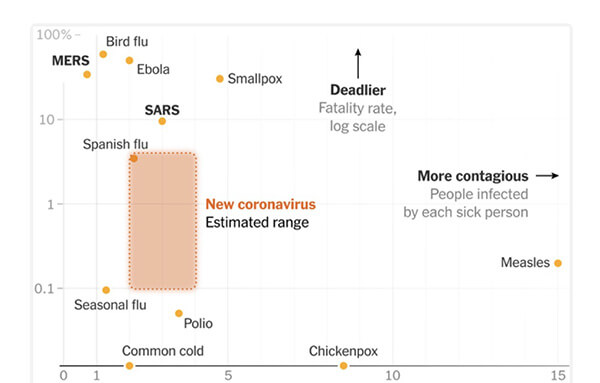
SARS-CoV-2 is more contagious than the flu, with each infected person spreading the disease to 1.5-3.5 people absent effective containment. The virus is not “air-borne” but spreads through droplet contact.
COVID-19 is considered more severe than the regular flu which has an average mortality rate of 0.1% in the overall population. Certain sections of the population are more vulnerable.
WHAT ARE THE SYMPTOMS?
Symptoms of COVID-19 are flu-like, with dry cough and possible develop shortness of breath. Other symptoms include fever, muscle aches, and generally feeling crappy. Sniffles and sneezing are less common.
So far, 80% of infected people have a mild case of the illness, and many (including children) are asymptomatic.
People over 60, patients who are immune-compromised, or who have other underlying conditions such as cardiovascular disease or chronic respiratory diseases are significantly more vulnerable. Pneumonia is common in this segment of the population.
WHAT SHOULD YOU DO?
Wash your hands often for 20 seconds each. A face mask is not recommended if you’re not feeling sick.
If you feel sick: Stay away from crowded areas and avoid public transport. Rest and hydrate. Stay home if possible until your symptoms pass.
If you’re over 60 or have other at-risk symptoms: Talk to your provider.
The C.D.C. has warned older and at-risk travelers to avoid Japan, Italy and Iran. The agency also has advised against all nonessential travel to South Korea and China.
IS THERE A TEST? A VACCINE?
Current testing takes 36-48 hours to provide a result.
A rapid swab test is expected to be available within the coming weeks.
There is no approved antiviral drug for COVID-19, though several are being tested.
An experimental vaccine for COVID-19 may be ready for testing in humans within a few months, but will take much longer, at least a year, to become available for widespread use.





how to buy clomid on line